Tiêu đề bài báo: Granulocyte colony-stimulating factor reduces biliary fibrosis and ductular reaction in a mouse model of chronic cholestasis
Tạp chí: Liver Research, số tháng 3 năm 2023
Toàn văn: https://www.sciencedirect.com/science/article/pii/S2542568423000053
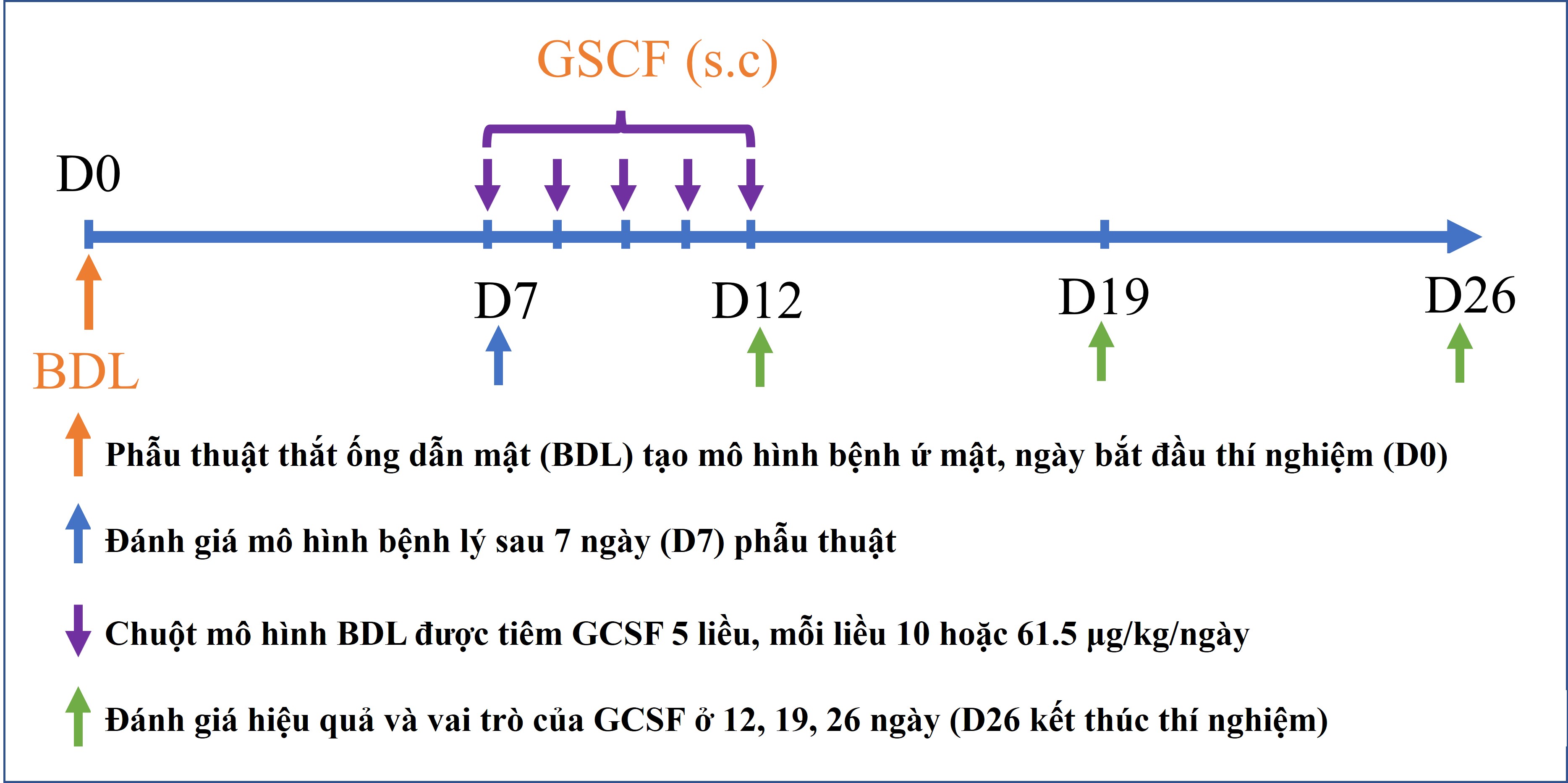
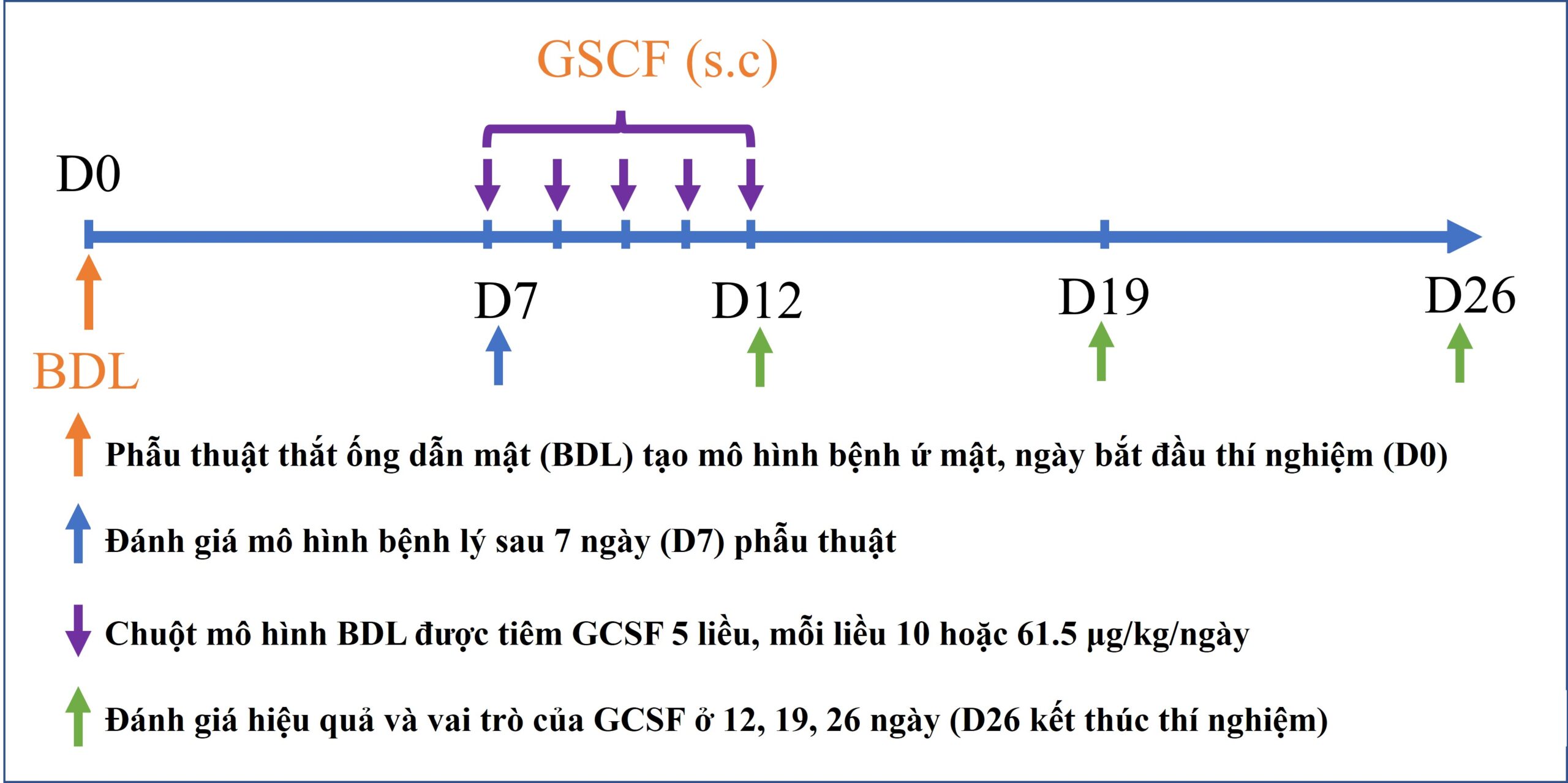
Nhân tố kích thích bạch cầu hạt (GCSF) là protein của cơ thể tác động lên tế bào gốc tủy xương tăng sinh, phân chia và di cư. Đây là thuốc được chỉ định điều trị thường quy cho các bệnh nhân bị suy bạch cầu ở người. Đồng thời, GCSF củng được ứng dụng trong việc tăng cường huy động tế bào gốc ra ngoài máu ngoại vi để thu nhận và cấy ghép cho bệnh nhân ở nhiều nghiên cứu khác nhau. Trong nghiên cứu này, nhóm tác giả đã sử dụng GCSF để ứng dụng điều trị mô hình chuột bị bệnh lý gan do tắc nghẽ đường mật, mô hình mô phỏng bệnh lý hẹp đường mật ở người.
Hiện nay, hẹp đường mật là một bệnh lý có tình trạng diễn tiến nhanh với tỷ lệ tử vong và tái phát cao sau điều trị đặt ra yêu cầu về việc phát triển liệu pháp mới giúp hỗ trợ điều trị, kéo dài thời gian sống cho bệnh nhân là rất cần thiết. GCSF đã được chứng minh có nhiều vai trò giúp hỗ trợ điều trị trên các bệnh lý gan giai đoạn cuối như tăng cường sự di cư của tế bào gốc về mô tổn thương, kích thích sự tăng sinh tế bào gan và điều hòa đáp ứng miễn dịch. Do đó, nhóm nghiên cứu tái tạo gan (Hepatoregeneration) tiến hành nghiên cứu về hiệu quả và vai trò của GCSF trên mô hình bệnh lý gan mạn tính do ứ mật.
Kết quả của công bố cho thấy, hiệu quả điều trị bệnh gan mạn tính do ứ mật của GCSF phụ thuộc vào liều lượng thuốc sử dụng. Đồng thời, nghiên cứu cũng chỉ ra các cơ chế tiềm năng của GCSF trong điều trị bệnh gan trên mô hình tắc nghẽn đường mật này gồm (i) tăng cường huy động tế bào gốc di cư về gan; (ii) kích thích sự tăng sinh tế bào gan; (iii) ức chế tế bào gây xơ là nguyên bào sợi cửa; and (iv) ức chế phản ứng ống mật. Các kết quả này là cơ sở cho việc ứng dụng GCSF trên lâm sàng trong điều trị bệnh lý hẹp đường mật.
Hepatoregeneration, PTN Nghiên cứu và Ứng dụng Tế bào gốc, Trường đại học Khoa học Tự nhiên, Đại học Quốc gia Hồ Chí Minh.